|
FENTIN CHLORIDE |
|
PRODUCT
IDENTIFICATION
|
| CAS
NO. |
639-58-7 |
|
| EINECS
NO. |
211-358-4 |
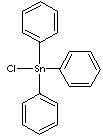
| FORMULA |
(C6H5)3SnCl |
| MOL
WT. |
385.46 |
|
H.S.
CODE
|
|
| TOXICITY |
Oral rat LD50: 135 mg/kg |
| SYNONYMS |
Chlorotriphenylstannane; Phenostat-C;
|
| Chlorotriphenyltin; Triphenylhydroxytin; Triphenylstannium chloride; Triphenyltin chloride;
Tinmate; TPTC;
|
| DERIVATION |
|
|
CLASSIFICATION
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
white
crystalline
powder |
| MELTING POINT |
97 - 107 C |
| BOILING
POINT |
240 C at 13.5
mmHg |
| SPECIFIC GRAVITY |
|
| SOLUBILITY
IN WATER |
Insoluble |
| pH |
|
| VAPOR DENSITY |
|
|
REFRACTIVE
INDEX
|
|
|
NFPA
RATINGS
|
|
|
AUTOIGNITION
|
|
| FLASH
POINT |
|
| STABILITY |
Decomposes under
sunlight |
|
GENERAL
DESCRIPTION & APPLICATIONS
|
|
Tin
compounds are classified into two main groups;
inorganic-tin and organo-tin compounds. The organo-tin
compounds are defined as compounds in which at least
one tin-to-carbon bond exist. But the inorganic-tin
compounds do not contain carbon as the principal element.
Inorganic-tin compounds are relatively simple in their
molecular structure and, like tin itself, are not considered
to be toxic. Tin atoms can replace carbon atoms in chemical
compounds, and a great variety of organo-tin compounds
are known.
INORGANIC TIN COMPOUNDS
The
largest use for inorganic tin compounds is in electrolytes
for plating tin and tin alloys. The more important plating
chemicals are chlorides, sulfates, and fluoroborates
in acidic electrolytes and stannates in alkaline solutions.
Inorganic-tin compounds are divided into two series:
stannous, or tin(II), compounds and stannic, or tin(IV),
compounds.Chemically, tin exhibits valencies of 2 and
4. It resists attack by water but is dissolved by strong
acids and alkalis. One of common compounds of tin(II)
are stannous chloride (SnCl2) used in tin galvanizing,
as a reducing agent in the manufacture of polymers and
as a mordant in dyeing.; stannous oxide (SnO) employed
in making tin salts for chemical reagents and for plating;
and stannous fluoride (SnF2) is the additive in fluoride
tooth-pastes. Inorganic tin chemicals are used as catalysts
in a number of industrial processes. stannous octoate
is the catalyst that produces the foaming action that
turns the liquid plastic into a foamlike solid structure
in the manufacture of polyurethane foam. Tin(IV) compounds
of significance include stannic chloride (SnCl4) is
widely used as a stabilizer for perfumes and as a starting
material for other tin salts; and stannic oxide(SnO2)
is a useful catalyst in certain industrial processes
and a polishing powder for steel. Tin sulfide is used
as a bronzing agent for wood colouring
ORGANOTIN
COMPOUNDS
The greatest use of di-organotin compounds is stabilizers in the
manufacture of polyvinyl chloride, or PVC. The particular importance of these
di-organotins lies in their outstanding ability to preserve the clarity and
transparency of PVC, not only when being processed but also in subsequent
service. Organotin-stabilized PVC is used in water pipes and in food packaging
applications as tin compounds used in these applications are known as nontoxic.
In contrast to the nontoxic compounds employed as stabilizers, some
tri-organotin compounds (e.g., tributyl- and triphenyltins) are powerful
biocides and have found use in a number of relevant applications, such as
fungicide, algicide, molluscicide in underwater and anti-fouling paints
extensively, preservatives for wood, as slimicide on masonry, as biocide
disinfectant for textile and leather processing, cooling system, pulp and paper
mill and brewery. The tributyltin family or fentine (triphenyltin) chemicals
include;
- Tributyltin benzoate (CAS RN: 4342-36-3)
- Tributyltin
chloride (CAS RN: 1461-22-9)
- Tributyltin
fluoride (CAS RN: 1983-10-4)
- Tributyltin
linoleate (CAS RN: 24124-25-2)
- Tributyltin
methacrylate (CAS RN: 2155-70-6)
- Tributyltin
naphthenate (CAS RN: 85409-17-2)
- Tributyltin
oxide (CAS RN: 56-35-9)
- Tributyltin sulfide (CAS
RN: 4808-30-4)
- Tributyltin adipate (CAS RN: 7437-35-6)
- Tributyltin acetate
(CAS RN: 56-36-0)
- Triphenyltin hydroxide (CAS RN: 76-87-9)
- Triphenyltin
acetate (CAS RN: 900-95-8)
- Triphenyltin chloride (CAS RN: 639-58-7)
Tributyltin compounds are usually clear to yellowish liquids
with an unpleasant odor. Triphenyltincompounds are white solids with low vapour
pressures. Tri-organotin compounds are derivatives of tetravalent tin. They are
lipophilic and have low water solubility. Physical and chemical properties of tri-organotin compounds vary
depending
upon the anion linked to tin. Tributyltin derivatives have toxic
properties to gram positive bacteria are used as disinfectants on surfaces such
as hospital floors and sports arenas, combined with gram negative bactericides.
Tin chemicals also used as flame retardants to treat fabrics and plastics. Tributyltin methacrylate is used as a stabiliser for
PVC.
Other industrial applications of organotin
compounds include as rodent repellents, antioxidants, curing agents
and corrosion inhibitors.
|
| SALES
SPECIFICATION |
|
APPEARANCE
|
white
powder |
|
ASSAY |
95.0%
min
|
|
MELTING POINT |
97- 107 C |
| TRANSPORTATION |
| PACKING |
25kgs
in fiber drum |
| HAZARD CLASS |
6.1 (Packing
Group: III) |
| UN
NO. |
3146 |
| OTHER
INFORMATION |
|
Hazard Symbols: T
N, Risk Phrases: 23/24/25-50/53, Safety Phrases:
26-27-28-45-60-61 |
|
STANNIC
& STANNOUS COMPOUNDS
|
|
Chemically, tin exhibits
valencies of 2 and 4. Inorganic-tin compounds are divided into
two series: stannous, or tin(II), compounds which
contain tin as a quadrivalent element,
whereas
stannic, or tin(IV), compounds containing tin as a bivalent element.
- STANNIC CHLORIDE
[SnCI4 ,
CAS RN: 7646-78-8
(Anhydrous), 10025-69-1
(Dihydrate)]:
a caustic liquid;
soluble in water (decomposes in hot water), alcohol,
carbon disulfide and turpentine oil; boiling point
114 c; toxic and corrosive if inhaled or spilled
on the skin; used as an intermediate
in the manufacture of organo-tin compounds ; in soaps as a colour and perfume
stabilizer and bacteria and fungi control; as a catalyst in the polymerisation
of styrene; textile finishing; glass: strengthening; eletroconductive and
electroluminescent surface coatings; manufacture of fuchsin, colour lakes,
ceramics; stabilizer for resins; manufacture of blue print and other sensitized
papers; also known as tin chloride; tin tetrachloride.
- STANNIC OXIDE
[SnO2 ,CAS
RN: 18282-10-5]:
a white powder
compound;
insoluble in water, soluble in concentrated sulfuric acid; melting point 1127
C; found in nature as the mineral
cassiterite, or prepared by the reaction between tin and concentrated nitric
acid at high temperatures; used as a polishing agent for glass, metals, and
metallic dental restorations, and as a
catalyst; also known as flowers of tin; stannic acid; stannic anhydride; tin
dioxide; tin oxide; tin peroxide.
- STANNIC BROMIDE [SnBr4,
CAS RN: 7789-67-5]:
a white crystalline compound; soluble in water and
alcohol; melting point 31 C; fumed when exposed to air;
used in mineral separations.; also known as tin tetrabromide.
- STANNIC CHROMATE [Sn(CrO4)2];
a yellow to brownish toxic crystals; slightly soluble in water;
used as a polishing agent for porcelain and china.
- STANNIC IODIDE [SnI4, CAS RN: 7790-47-8]
a yellow to reddish crystals; decomposes in water, soluble in alcohol, ether,
chloroform, carbon disulfide, and benzene; melt point 144 C; sublime at 180
C; also known as tin tetraiodide.
- STANNIC SULFIDE [SnS2,
CAS RN: 1315-01-1]:
a
yellow to brown powder; insoluble in water, soluble in alkaline sulfides;
decomposes at red heat; used as a pigment and for imitation gilding. Also known
as artificial gold; mosaic gold; tin bisulfide.
- TIN DICHLORIDE BIS(2,4-PENTANEDIONATE)
[SnC10H14Cl2O4,
CAS RN: 16919-46-3]
- TIN (IV) PHTHALOCYANINE
DICHLORIDE [CAS RN: 18253-54-8]
- TIN(IV) ACETATE
[SnC8H12O8,
CAS RN: 2800-96-6]:
Tetraacetoxytin
- TIN(IV)T-BUTOXIDE
[SnC16H36O4,
CAS RN: 36809-75-3]
- DI-N-BUTYL
TIN(IV) DICHLORIDE [SnC16H36O4,
CAS RN: 683-18-1]:
Dibutyltin dichloride
- TIN (IV) METHACRYLATE
[SnC16H20O8,
CAS RN: 69064-21-7]
- TIN(IV)
FLUORIDE [SnF4,
CAS RN: 7783-62-2]:
Tin tetrafluoride
- TIN(IV) BROMIDE
[SnBr4,
CAS RN: 7789-67-5]:
Tin tetrabromide
- STANNIC
PHOSPHIDE
- STANNOUS CHLORIDE
[SnCl2, CAS RN: 7772-99-8
(anhydrous), 7772-99-8
(dihydrate)] : a white crystal; soluble in water, alcohol, and alkalies; melting
point 247 C; used as lube oil
additive; tin galvanizing and as a reducing agent in the manufacture of polymers
and dyes and printing textiles; in the manufacture of stannous salts
(particularly the oxide, sulphate, octoate and 2- ethyl hexotate); in
manufacture of pharmaceuticals and fine chemicals as a reducing agent; as a
reducing agent in the extraction and purification of precious metals; surface
sensitizer prior to silvering in the manufacture of mirrors;
electroplating;
- STANNOUS FLUORIDE [SnF2,
CAS RN: 7783-47-3]:
used topically to the teeth as a dental caries
prophylactic; Also known
as tin difluoride.
- STANNOUS PYROPHOSPHATE
[Sn2P2O7, 15578-26-4]: a diagnostic aid used in
bone imaging. Tin (II) pyrophosphate.
- SODIUM STANNATE [Na2SnO3,
CAS RN: 12058-66-1
(Anhydrous) 12209-98-2 (Trihydrate) 12027-70-2
(Hexahydroxide)]:
white crystals; insoluble
in water, alcohol; used as a salt in alkaline tin plating electrolyte;
used in surface
coatings (paper), in manufacturing other metallic stannates and tin
oxide coatings; also known as preparing salt.
- STANNOUS 2-ETHYLHEXOATE [Sn(C8H15O2)2,
CAS RN: 301-10-0]:
a yellow liquid; soluble in benzene, toluene, and petroleum
ether; used as a lubricant, a vulcanizing agent, and a stabilizer for
transformer oil.
- STANNOUS BROMIDE [SnBr2,
CAS RN: 10031-24-0]:
a yellowish powder;
soluble in water, alcohol, acetone, ether, and dilute hydrochloric acid; darken
on exposure on air; melting point 215C; also known as tin dibromide.
- STANNOUS CHROMATE [SnCrO4]:
a brown powder; slightly soluble in water; used to make
porcelain and glass; also known as tin chromate.
- STANNOUS FLUORIDE [SnF2,
CAS RN: 7783-47-3]: a white, lustrous
powder; slightly soluble in water; used to fluoridate toothpaste and as a
medicine; also known as tin difluoride.
- STANNOUS
METHANESULFONATE [Sn(CH3SO3)2, CAS RN:
53408-94-9];
electroplating chemical
- STANNOUS
OXALATE [SnC2O4, CAS RN: 814-94-8,
17480-26-1]: aA white crystalline powder; decomposes
at 280 C; soluble in acids; used in textile dyeing and printing; also
known as tin oxalate.
- STANNOUS OXIDE [SnO,
CAS RN: 21651-19-4] a black powder; insoluble in water, soluble in acids and strong bases;
decomposes when heated; unstable in air; used as a reducing agent and chemical intermediate, and
for glass plating; also known as tin protoxide.
- STANNOUS SULFATE [SnSO4,
CAS RN: 7488-55-3]:
white to
yellowish crystalline powder; decomposes rapidly in water;
losing SO2 at 360 C;
used for dyeing and tin plating; also known as tin sulfate.
- STANNOUS SULFIDE
[SnS,
CAS RN: 1314-95-0]:
a dark crystals; insoluble in water, decomposes
in concentrated
hydrochloric acid; melt point 880 C; used as an analytical reagent and catalyst,
and in bearing material; also known as tin monosulfide.
- BARIUM STANNATE
[BaO3Sn,
CAS RN: 12009-18-6]
- CALCIUM
STANNATE [CaO3Sn,
CAS RN: 12013-46-6]
- COPPER (II) STANNATE
[CuO3Sn,
CAS RN: 12019-07-7]
- LEAD STANNATE, DIHYDRATE
[H4O5PbSn,
CAS RN: 12036-31-6]
- ZINC
STANNATE [CuO3Sn,
CAS RN: 12036-37-2]
- SODIUM STANNATE
[Na2O3Sn,
CAS RN: 12058-66-1
12209-98-2 (Trihydrate)]
- POTASSIUM STANNATE TRIHYDRATE
[H6K2O6Sn,
CAS RN: 12142-33-5]
- STRONTIUM
STANNATE [SrO3Sn,
CAS RN: 12143-34-9]
- COBALT(II)
STANNATE DIHYDRATE [CoH4O5Sn,
CAS RN: 1345-19-3]
- SODIUM
TRIFLUOROSTANNATE [NaF3Sn,
CAS RN: 13782-22-4]
- AMMONIUM HEXACHLOROSTANNATE(IV)
[H8N2Cl6Sn,
CAS RN: 16960-53-5]
- LITHIUM
HEXAFLUOROSTANNATE(IV) [Li2F6Sn,
CAS RN: 17029-16-2]
|
|
